Health Food Filing System in China
2024-06-28 19:39:48
What is the Health Food Filing System in China?
Health Food falling within the Health Food Raw Material Directory in China and belonging to supplementary nutrients such as vitamins and minerals must adhere to the filing system.
Why do these actions apply to the filing system?
According to Chinese laws and rules for Health Food, all enterprises producing and selling health food within China, as well as overseas enterprises exporting health food to China, are required to follow the regulations of the filing system.
What do you need to do?
As a Health Food producer or seller in China, you need to provide all required documents to prove the food is safe, with health functions, and has reasonable and steady quality.
Workflow
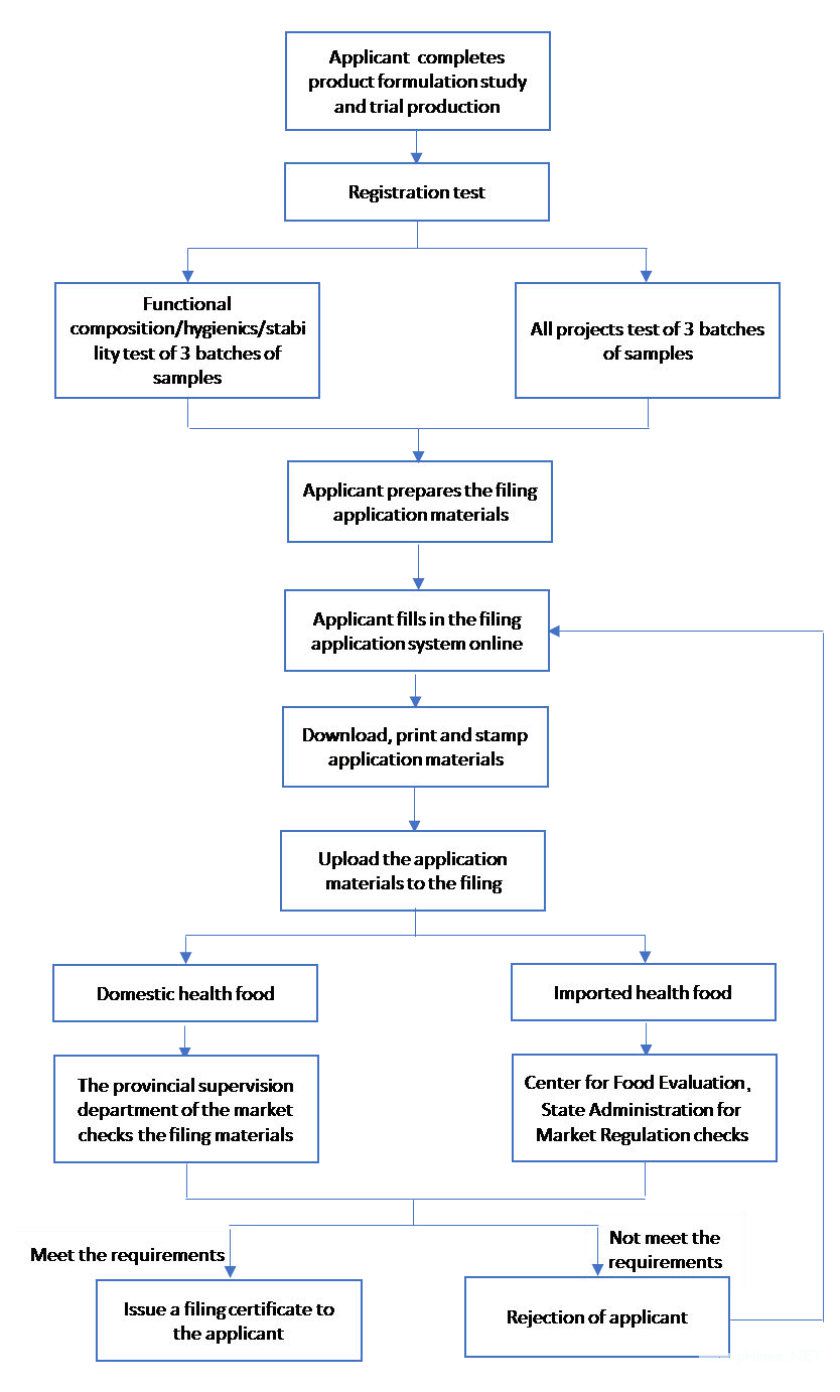
Document List
1. Application Form
2. legal responsibility commitment letter.
3. Business registration certificate.
4. Technical requirements for the product.
5. Product formulation documents.
6. Production process documents.
7. Inspection report.
8. Safety and health function evaluation documents.
9. Package materials information.
10. Label information.
11. Others regarding to safety and function.
12. Qualification certificate issued by oversea authorities.*
13. Evidence for sales in the country of production over 1year.*
14. Original package documents*
N.B marked * documents apply to enterprise in oversea.
What do we do for you?
1. Legal Advisory Services
(1)Legal and regulatory consultation on the Health Food Ingredients Filling System;
(2)Customized consultation on registration strategies and training for Health Food Ingredients;
2. Product Compliance Services
(1)Risk assessment for registration;
(2)Registration application services;
(3)Post-registration compliance services for production and operation activities.
Health Food falling within the Health Food Raw Material Directory in China and belonging to supplementary nutrients such as vitamins and minerals must adhere to the filing system.
Why do these actions apply to the filing system?
According to Chinese laws and rules for Health Food, all enterprises producing and selling health food within China, as well as overseas enterprises exporting health food to China, are required to follow the regulations of the filing system.
What do you need to do?
As a Health Food producer or seller in China, you need to provide all required documents to prove the food is safe, with health functions, and has reasonable and steady quality.
Workflow

Document List
1. Application Form
2. legal responsibility commitment letter.
3. Business registration certificate.
4. Technical requirements for the product.
5. Product formulation documents.
6. Production process documents.
7. Inspection report.
8. Safety and health function evaluation documents.
9. Package materials information.
10. Label information.
11. Others regarding to safety and function.
12. Qualification certificate issued by oversea authorities.*
13. Evidence for sales in the country of production over 1year.*
14. Original package documents*
N.B marked * documents apply to enterprise in oversea.
What do we do for you?
1. Legal Advisory Services
(1)Legal and regulatory consultation on the Health Food Ingredients Filling System;
(2)Customized consultation on registration strategies and training for Health Food Ingredients;
2. Product Compliance Services
(1)Risk assessment for registration;
(2)Registration application services;
(3)Post-registration compliance services for production and operation activities.





